Alginic acid-derived mesoporous carbon (Starbon®) as template and reducing agent for the hydrothermal synthesis of mesoporous LiMn2O4 grafted with carbonaceous species†
Abstract
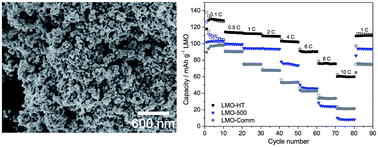
An alginic acid-derived mesoporous carbonaceous material (Starbon® A300) was used as a sacrificial porous template providing both a reducing environment and anchoring sites for LMO precursors, KMnO4 and LiOH. After hydrothermal treatment at 180 °C for 24 h, the resulting nanocrystalline LMO particles (≈40 nm) spontaneously aggregated, generating a mesoporous structure with a relatively high mesopore volume (≈0.33 cm3 g−1) and large pore size (≈30 nm). Moreover, a small amount (≈0.6 wt%) of residual carbon was present in this mesoporous LMO. This carbon, which arises from carbonaceous species grafted at the surface of the LMO nanoparticles, was found to significantly enhance the rate capability of LMO by reducing the internal electronic resistance. Finally, a “green” LMO electrode formulated using this Starbon-derived LMO as an active material, Starbon® A800 as a conductive additive and sodium alginate as a binder was tested, showing promising electrochemical performance.


 Please wait while we load your content...
Please wait while we load your content...