Surface termination effects on the oxygen reduction reaction rate at fuel cell cathodes†
Abstract
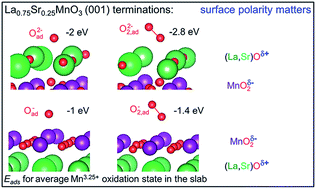
The results of first principles calculations of oxygen vacancy and oxygen adsorbate concentrations are analyzed and compared for the polar (La,Sr)O and MnO2 (001) terminations of (La,Sr)MnO3 fuel cell cathode materials. Both quantities strongly depend on the average Mn oxidation state (La/Sr ratio). In thin symmetrical slabs, the cation nonstoichiometry also plays an important role by modifying the average Mn oxidation state. The surface oxygen vacancy concentration for the (La,Sr)O termination is more than 5 orders of magnitude smaller when compared to the MnO2 termination. The vacancy and adsorbed oxygen migration energies as well as the dissociation barriers of adsorbed molecular oxygen species are determined. The encounter of adsorbed atomic oxygen and surface oxygen vacancy is identified as the rate determining step of the oxygen incorporation reaction. Since the increase of atomic and molecular oxygen adsorbate concentration is limited by the typical saturation level in the range of 20% for charged adsorbates, the overall oxygen incorporation rate is predicted to be significantly smaller for the (La,Sr)O termination.


 Please wait while we load your content...
Please wait while we load your content...