A full monolayer of superoxide: oxygen activation on the unmodified Ca3Ru2O7(001) surface†
Abstract
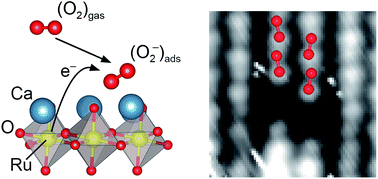
Activating the O2 molecule is at the heart of a variety of technological applications, most prominently in energy conversion schemes including solid oxide fuel cells, electrolysis, and catalysis. Perovskite oxides, both traditionally-used and novel formulations, are the prime candidates in established and emerging energy devices. This work shows that the as-cleaved and unmodified CaO-terminated (001) surface of Ca3Ru2O7, a Ruddlesden–Popper perovskite, supports a full monolayer of superoxide ions, O2−, when exposed to molecular O2. The electrons for activating the molecule are transferred from the subsurface RuO2 layer. Theoretical calculations using both, density functional theory (DFT) and more accurate methods (RPA), predict the adsorption of O2− with Eads = 0.72 eV and provide a thorough analysis of the charge transfer. Non-contact atomic force microscopy (nc-AFM) and scanning tunnelling microscopy (STM) are used to resolve single molecules and confirm the predicted adsorption structures. Local contact potential difference (LCPD) and X-ray photoelectron spectroscopy (XPS) measurements on the full monolayer of O2− confirm the negative charge state of the molecules. The present study reports the rare case of an oxide surface without dopants, defects, or low-coordinated sites readily activating molecular O2.
- This article is part of the themed collections: Celebrating Excellence in Research: Women of Materials Science and 2018 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...