Yolk–shell-structured MnO2 microspheres with oxygen vacancies for high-performance supercapacitors†
Abstract
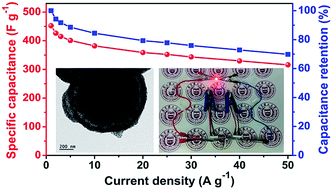
Yolk–shell-structured MnO2 microspheres with oxygen vacancies (ov-MnO2@MnO2) were successfully constructed by a facile three-step method. Morphological observations showed that the as-obtained ov-MnO2@MnO2 microspheres possessed distinctive yolk@void@shell configurations with an average diameter of 1.13 μm. Both the shell and yolk were assembled by a large amount of homogeneous MnO2 nanoparticles with an average diameter of 12 nm. The yolk–shell-structured ov-MnO2@MnO2 microsphere electrode exhibited a large specific surface area (259.83 m2 g−1) and good conductivity, thus it achieved high specific capacitance (452.4 F g−1 at 1 A g−1 and 316.1 F g−1 at 50 A g−1), excellent cycling stability (10 000 cycles) and superior rate capability (∼79.2% and 69.9% of the initial capacity at 20 A g−1 and 50 A g−1, respectively). It is noted that the asymmetric supercapacitor (ASC) composed of yolk–shell-structured ov-MnO2@MnO2 microspheres (as the positive electrode) and commercial activated carbon (as the negative electrode) can deliver a high energy density of 40.2 W h kg−1 and a maximum power density of 22.28 kW kg−1. The superior electrochemical performance of ov-MnO2@MnO2 is mainly ascribed to the unique yolk@void@shell nanostructure, the presence of oxygen vacancies in the crystal lattice and the synergistic effect of the individual components of the hybrid.


 Please wait while we load your content...
Please wait while we load your content...