Cyclodextrin-based complex coacervate core micelles with tuneable supramolecular host–guest, metal-to-ligand and charge interactions†
Abstract
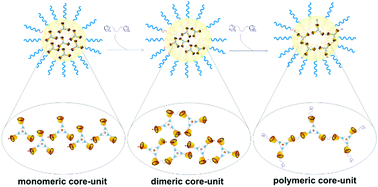
Micelles have been recognized as versatile platforms for different biomedical applications, from bioimaging to drug delivery. Complex coacervate core micelles present great advantages compared to traditional micelles, however controlling the number of charges per core-unit and the stability is still a challenge. We here present cyclodextrin-based complex coacervate core micelles where the charge per core-unit can be straightforwardly tuned by cyclodextrin host–guest interactions. By varying the ratio between two adamantane guest molecules, 1-adamantanecarboxylic acid and 1,3-adamantanediacetic acid, the charge of the monomeric core-units can be finely tuned from 6− to 9−. By adding an adamantane bislinker, monomeric core-units can be combined together in dimeric and polymeric structures, increasing the micelles’ stability. The orthogonal supramolecular host–guest and coordination-chemistry allows for well-controlled cyclodextrin-based complex coacervate core micelles that offer a versatile platform for designing future, e.g., responsive systems.


 Please wait while we load your content...
Please wait while we load your content...