Selective C–H halogenation over hydroxylation by non-heme iron(iv)-oxo†
Abstract
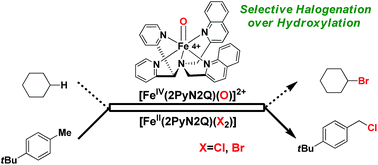
Non-heme iron based halogenase enzymes promote selective halogenation of the sp3-C–H bond through iron(IV)-oxo-halide active species. During halogenation, competitive hydroxylation can be prevented completely in enzymatic systems. However, synthetic iron(IV)-oxo-halide intermediates often result in a mixture of halogenation and hydroxylation products. In this report, we have developed a new synthetic strategy by employing non-heme iron based complexes for selective sp3-C–H halogenation by overriding hydroxylation. A room temperature stable, iron(IV)-oxo complex, [Fe(2PyN2Q)(O)]2+ was directed for hydrogen atom abstraction (HAA) from aliphatic substrates and the iron(II)-halide [FeII(2PyN2Q)(X)]+ (X, halogen) was exploited in conjunction to deliver the halogen atom to the ensuing carbon centered radical. Despite iron(IV)-oxo being an effective promoter of hydroxylation of aliphatic substrates, the perfect interplay of HAA and halogen atom transfer in this work leads to the halogenation product selectively by diverting the hydroxylation pathway. Experimental studies outline the mechanistic details of the iron(IV)-oxo mediated halogenation reactions. A kinetic isotope study between PhCH3 and C6D5CD3 showed a value of 13.5 that supports the initial HAA step as the RDS during halogenation. Successful implementation of this new strategy led to the establishment of a functional mimic of non-heme halogenase enzymes with an excellent selectivity for halogenation over hydroxylation. Detailed theoretical studies based on density functional methods reveal how the small difference in the ligand design leads to a large difference in the electronic structure of the [Fe(2PyN2Q)(O)]2+ species. Both experimental and computational studies suggest that the halide rebound process of the cage escaped radical with iron(III)-halide is energetically favorable compared to iron(III)-hydroxide and it brings in selective formation of halogenation products over hydroxylation.
- This article is part of the themed collections: Celebrating the Chemical Sciences in India - Leaders in the Field Symposium 2020, Most popular 2018-2019 chemical biology articles and 2019 International Open Access Week Collection


 Please wait while we load your content...
Please wait while we load your content...