Embryonic brass: pseudo two electron Cu/Zn clusters†
Abstract
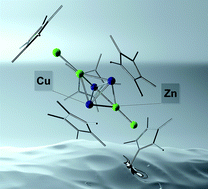
The isoelectronic M7 clusters [Cu3Zn4](Cp*)5 (1) and {[Cu2Zn5](Cp*)5}+ (2) are described. While 1 can be isolated only as a minor side product from the reaction of Cu(CH3CO2) with equimolar amounts of [Zn2Cp*2] with the trigonal cluster [CuZn2](Cp*)3 as the major product, 2 is available in acceptable yields from the reaction of [CuZn2](Cp*)3 with the Cp*Zn2-transfer-reagent [Cp*Zn2(Et2O)3][BAr4F]. The trigonal bipyramidal Cu/Zn-clusters exhibit exceptional bonding situations: with formally only one skeleton electron pair they can be regarded as highly electron deficient. However, a detailed DFT analysis reveals that the cluster bonding is supported by 3d orbital contributions of the trigonal metal base unit. The data contribute to the development of an advanced tool-box for synthesis of Hume-Rothery intermetallic (e.g. brass) inspired clusters.


 Please wait while we load your content...
Please wait while we load your content...