A vesicle-aggregation-assembly approach to highly ordered mesoporous γ-alumina microspheres with shifted double-diamond networks†
Abstract
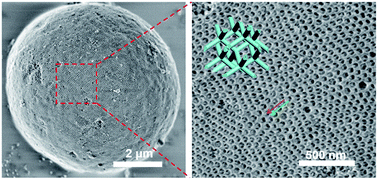
Alumina materials have widely been used in industrial fields, such as catalysis and adsorption. However, due to the fast sol–gel process and complicated crystalline-phase transformation, the synthesis of alumina materials with both highly ordered mesostructures and crystallized frameworks remains a great challenge. Herein, we report a novel vesicle-aggregation-assembly strategy to prepare highly ordered mesoporous γ-alumina microspheres with unique shifted double-diamond networks for the first time, by using diblock copolymer poly(ethylene oxide)-b-poly(methyl methacrylate) (PEO-b-PMMA) as a template and aluminum isopropoxide as a precursor in a tetrahydrofuran (THF)/hydrochloric acid binary solvent. During the gradual evaporation of THF and H2O, the as-made Al3+-based gel/PEO-b-PMMA composites can be obtained through a co-assembly process based on the hydrogen bonding interaction between hydroxyl groups of alumina oligomers and PEO segments of the diblock copolymers. The formed composites exhibit a spherical morphology with a wide size distribution (diameter size 1–12 μm). Furthermore, these composite microspheres possess an inverse bicontinuous cubic mesostructure (double diamond, Pn![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) with Al3+-based gel buried in the PEO-b-PMMA matrix in the form of two intertwined but disconnected networks. After a simple calcination at 900 °C in air, the structure of the resultant mesoporous alumina changes to a relatively low symmetry (shifted double diamond, Fd
m) with Al3+-based gel buried in the PEO-b-PMMA matrix in the form of two intertwined but disconnected networks. After a simple calcination at 900 °C in air, the structure of the resultant mesoporous alumina changes to a relatively low symmetry (shifted double diamond, Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m), ascribed to the shifting of the two alumina networks due to loss of the templates. Meanwhile, the unit cell size of the alumina mesostructure decreases from ∼131 to ∼95 nm. The obtained ordered mesoporous alumina products retain the spherical morphology and possess ultra-large mesopores (∼72.8 nm), columnar frameworks composed of γ-alumina nanocrystalline particles (crystal size of ∼15 nm) and high thermal stability (up to 900 °C). As a support of Au nanoparticles, the formed Au/mesoporous γ-alumina composite catalysts have been used in the catalytic reduction of 4-nitrophenol with a high kinetic constant k of 0.0888 min−1, implying promising potential as a catalyst support.
m), ascribed to the shifting of the two alumina networks due to loss of the templates. Meanwhile, the unit cell size of the alumina mesostructure decreases from ∼131 to ∼95 nm. The obtained ordered mesoporous alumina products retain the spherical morphology and possess ultra-large mesopores (∼72.8 nm), columnar frameworks composed of γ-alumina nanocrystalline particles (crystal size of ∼15 nm) and high thermal stability (up to 900 °C). As a support of Au nanoparticles, the formed Au/mesoporous γ-alumina composite catalysts have been used in the catalytic reduction of 4-nitrophenol with a high kinetic constant k of 0.0888 min−1, implying promising potential as a catalyst support.
- This article is part of the themed collection: Editor’s Choice – Jihong Yu


 Please wait while we load your content...
Please wait while we load your content...