A chemical reporter facilitates the detection and identification of lysine HMGylation on histones†
Abstract
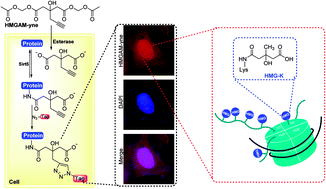
Lysine 3-hydroxyl-3-methylglutarylation (HMG-K) is a newly identified PTM that can occur non-enzymatically in mitochondria. However, the substrate scope of this new PTM remains insufficiently explored, which has greatly hindered the progress in interpreting its regulatory mechanisms and cellular functions. Here, we report the development of an alkyne-functionalized chemical reporter (HMGAM-yne), for the detection and identification of cellular HMGylated proteins. HMGAM-yne is cell-permeable and metabolically incorporated into proteins in living cells. Subsequent biorthogonal conjugation enables fluorescence visualization and identification of the protein substrates of HMG-K. Using HMGAM-yne, we also identified Sirt5 as an ‘eraser’ that regulates HMGylation in cells. In addition to the known mitochondrial HMG-K proteins, HMGAM-yne facilitates the discovery of multiple nuclear proteins, including histones, as novel substrates of lysine HMGylation.


 Please wait while we load your content...
Please wait while we load your content...