Visible light-promoted ring-opening functionalization of unstrained cycloalkanols via inert C–C bond scission†‡
Abstract
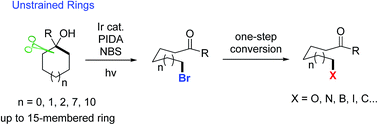
Described herein is a novel, useful, visible light-promoted ring-opening functionalization of unstrained cycloalkanols. Upon scission of an inert cyclic C–C σ-bond, a set of medium- and large-sized rings are readily brominated under mild reaction conditions to afford the corresponding distal bromo-substituted alkyl ketones that are hard to synthesize otherwise. The products are versatile building blocks, which are easily converted to other valuable molecules in one-step operation. This protocol is also applicable to the unprecedented ring-opening cyanation and alkynylation of unstrained cycloalkanols.
- This article is part of the themed collections: SU 120: Celebrating 120 Years of Soochow University and Most popular 2018-2019 organic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...