Dewetting transitions coupled to K-channel activation in cytochrome c oxidase†
Abstract
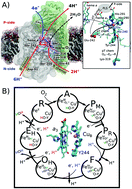
Cytochrome c oxidase (CcO) drives aerobic respiratory chains in all organisms by transducing the free energy from oxygen reduction into an electrochemical proton gradient across a biological membrane. CcO employs the so-called D- and K-channels for proton uptake, but the molecular mechanism for activation of the K-channel has remained elusive for decades. We show here by combining large-scale atomistic molecular simulations with graph-theoretical water network analysis, and hybrid quantum/classical (QM/MM) free energy calculations, that the K-channel is activated by formation of a reactive oxidized intermediate in the binuclear heme a3/CuB active site. This state induces electrostatic, hydration, and conformational changes that lower the barrier for proton transfer along the K-channel by dewetting pathways that connect the D-channel with the active site. Our combined results reconcile previous experimental findings and indicate that water dynamics plays a decisive role in the proton pumping machinery in CcO.


 Please wait while we load your content...
Please wait while we load your content...