A “waiting” carbon nitride radical anion: a charge storage material and key intermediate in direct C–H thiolation of methylarenes using elemental sulfur as the “S”-source†
Abstract
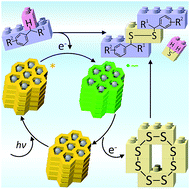
Potassium poly(heptazine imide), a carbon nitride based semiconductor with high structural order and a valence band potential of +2.2 V vs. NHE, in the presence of hole scavengers and under visible light irradiation gives the corresponding polymeric radical anion, in which the specific density of unpaired electrons reaches 112 μmol g−1. The obtained polymeric radical anion is stable under anaerobic conditions for several hours. It was characterized using UV-vis absorption, time resolved and steady state photoluminescence spectra. The electronic structure of the polymeric radical anion was confirmed by DFT cluster modelling. The unique properties of potassium poly(heptazine imide) for storing charges were employed in visible light photocatalysis. A series of substituted dibenzyldisulfanes was synthesized in 41–67% yield from the corresponding methylarenes via cleavage of the methyl C–H bond under visible light irradiation and metal-free conditions.


 Please wait while we load your content...
Please wait while we load your content...