XBphos-Rh: a halogen-bond assembled supramolecular catalyst†
Abstract
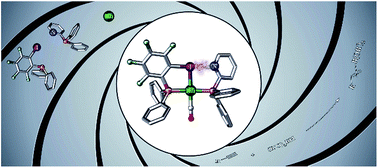
The use of halogen bonding as a tool to construct a catalyst backbone is reported. Specifically, pyridyl- and iodotetrafluoroaryl-substituted phosphines were assembled in the presence of a rhodium(I) precursor to form the corresponding halogen-bonded complex XBphos-Rh. The presence of fluorine substituents at the iodo-containing supramolecular motif was not necessary for halogen bonding to occur due to the template effect exerted by the rhodium center during formation of the halogen-bonded complex. The halogen-bonded supramolecular complexes were successfully tested in the catalytic hydroboration of terminal alkynes.
- This article is part of the themed collection: Most popular 2018-2019 supramolecular chemistry articles


 Please wait while we load your content...
Please wait while we load your content...