Asymmetric synthesis of multiple quaternary stereocentre-containing cyclopentyls by oxazolidinone-promoted Nazarov cyclizations†
Abstract
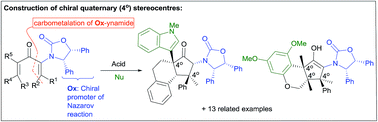
Carbometalation of oxazolidinone (Ox)-substituted ynamides is used to generate highly substituted Ox-divinyl (and aryl vinyl) ketones for use in Nazarov cyclizations. The Ox-group serves as a remarkably effective chiral activating group, enabling the torquoselective Nazarov cyclization of these sterically congested substrates to be performed under mild conditions. It also serves as a charge-stabilizing group in the intermediate oxyallyl cation, suppressing undesired [1,2]-sigmatropic shifts of neighboring substituents and facilitating the regio- and stereoselective incorporation of nucleophiles to yield cyclopentanoids containing up to three contiguous all-carbon quaternary (4°) stereocentres.


 Please wait while we load your content...
Please wait while we load your content...