Metal alkyls programmed to generate metal alkylidenes by α-H abstraction: prognosis from NMR chemical shift†
Abstract
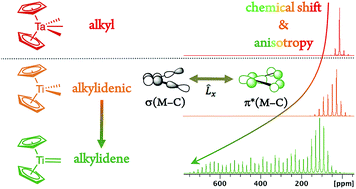
Metal alkylidenes, which are key organometallic intermediates in reactions such as olefination or alkene and alkane metathesis, are typically generated from metal dialkyl compounds [M](CH2R)2 that show distinctively deshielded chemical shifts for their α-carbons. Experimental solid-state NMR measurements combined with DFT/ZORA calculations and a chemical shift tensor analysis reveal that this remarkable deshielding originates from an empty metal d-orbital oriented in the M–Cα–Cα′ plane, interacting with the Cα p-orbital lying in the same plane. This π-type interaction inscribes some alkylidene character into Cα that favors alkylidene generation via α-H abstraction. The extent of the deshielding and the anisotropy of the alkyl chemical shift tensors distinguishes [M](CH2R)2 compounds that form alkylidenes from those that do not, relating the reactivity to molecular orbitals of the respective molecules. The α-carbon chemical shifts and tensor orientations thus predict the reactivity of metal alkyl compounds towards alkylidene generation.
- This article is part of the themed collection: Most popular 2018-2019 main group, inorganic and organometallic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...