Development of a selective, solvent-free epoxidation of limonene using hydrogen peroxide and a tungsten-based catalyst
Abstract
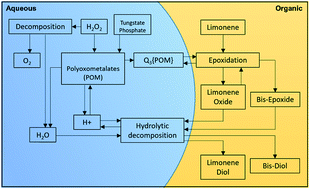
The development of a limonene epoxidation process using environment-friendly H2O2, with high H2O2 conversion (∼95%) and selectivity to the epoxide (100%), is reported in this paper. Parametric studies of temperature, oxidant, solvent, acid concentration and sodium sulphate amounts were performed with the focus on establishing a rapid and highly selective process. Approximately 95% conversion of H2O2 at 100% selectivity to limonene-1,2-epoxide was achieved in 15 minutes with a single-step addition of oxidant. The operating conditions included a 323 K temperature in a solvent-free environment, with a limonene/H2O2/catalyst molar ratio of 4 : 1 : 0.005, using a tungsten-based polyoxometalates. To prevent the hydrolysis of the epoxide, the reaction mixture was saturated with sodium sulphate. An acid concentration of lower than 0.04 M was used and found to have significant effect on the selectivity. Kinetic studies were performed to allow modelling of the reaction scheme. The activation energy was determined to be ∼36 kJ mol−1.


 Please wait while we load your content...
Please wait while we load your content...