Extension and functionalization of an encapsulating macrobicyclic ligand using palladium-catalyzed Suzuki–Miyaura and Sonogashira reactions of iron(ii) dihalogenoclathrochelates with inherent halogen substituents†‡
Abstract
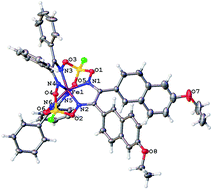
A new approach for performing Suzuki–Miyaura and Sonogashira reactions of iron(II) dihalogenoclathrochelates, optimizing their reaction conditions (such as temperature, solvent and a palladium-containing catalyst) and the nature of other reagents (such as arylboron components) is elaborated. These palladium-catalyzed reactions are very sensitive to the nature of the macrobicyclic substrates. The reactivity of the leaving halogen atoms correlates with their ability to undergo an oxidative addition, decreasing in the order: I > Br > Cl, and iron(II) diiodoclathrochelate underwent these C–C cross-couplings under their “classical” conditions. Phenylboronic, 4-carboxyphenylboronic and 6-ethoxy-2-naphthylboronic acids, and the diethyl ether of 4-(ethoxycarbonyl)boronic acid were tested as components of Suzuki–Miyaura reactions in DMF and in THF. The highest yields of the target products were obtained in DMF, while the highest activation was observed with sodium and potassium carbonates. The Suzuki–Miyaura reaction of a diiodoclathrochelate with 6-ethoxy-2-naphthylboronic acid gave the mono- and difunctionalized clathrochelates resulting from the tandem hydrodeiodination – C–C cross-coupling and double C–C cross-coupling reactions, respectively. Its Sonogashira reactions with trimethylsilylacetylene and acetylenecarboxylic acid in THF and in DMF were tested. This palladium-catalyzed reaction with a (CH3)3Si-containing active component gave the target products in a high total yield. The complexes obtained were characterized using elemental analysis, MALDI-TOF, UV-Vis, 1H and 13C{1H} NMR spectroscopy, and by single crystal XRD. Despite the non-equivalence of the ribbed α-dioximate fragments of their molecules, the encapsulated iron(II) ion is situated almost in the centre of its FeN6-coordination polyhedron, the geometry of which is almost intermediate between a trigonal prism and a trigonal antiprism.


 Please wait while we load your content...
Please wait while we load your content...