Aromatic fluorocopolymers based on α-(difluoromethyl)styrene and styrene: synthesis, characterization, and thermal and surface properties†
Abstract
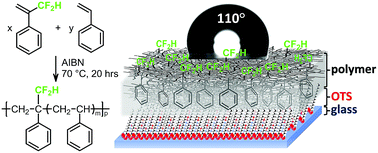
A study on the α-(difluoromethyl)styrene (DFMST) reactivity under conventional radical copolymerization conditions is presented. Although the homopolymerization of DFMST failed, its radical bulk copolymerization with styrene (ST) led to the synthesis of fluorinated aromatic polymers (FAPs). The resulting novel poly(DFMST-co-ST) copolymers were characterized by 1H, 19F and 13C NMR spectroscopies that evidenced the successful incorporation of DFMST units into copolymers and enabled the assessment of their respective molar percentages (10.4–48.2 mol%). The molar masses were in the range of 1900–17 200 g mol−1. The bulkier CF2H group in the α-position induced the lower reactivity of the DFMST comonomer. ST and DFMST monomer reactivity ratios (rDFMST = 0.0 and rST = 0.70 ± 0.05 at 70 °C) were determined based on linear least-square methods. These values indicate that DFMST monomer is less reactive than ST, retards the polymerization rate, and thus reduces the molar masses. Moreover, the thermal properties (Tg, Td) of the resulting copolymers indicate that the presence of DFMST units incorporated into poly(ST) structure promotes an increase of the Tg values up to 109 °C and a slightly better thermal stability than that of poly(ST). Additionally, the thermal decomposition of poly(DFMST-co-ST) copolymer (10.4/89.6) was assessed by simultaneous thermal analysis coupled with Fourier-transform infrared spectroscopy and thermogravimetric analysis coupled with mass spectrometry showing that H2O, CO2, CO and styrene were released. The surface analysis was focused on the effects of the –CF2H group at the α-position of styrene comonomers on surface free energy of the copolymer films. Water and diiodomethane contact angle (CA) measurements confirmed that these copolymers (Mn = 2300–17 200 g mol−1) are not exactly the same as polystyrenes (Mn = 2100–21 600 g mol−1) in the solid state. The CA hysteresis for poly(ST) (6–8°) and poly(DFMST-co-ST) copolymers (3–5°) reflected these differences even more accurately.


 Please wait while we load your content...
Please wait while we load your content...