Luminescence properties of ZnGa2O4:Cr3+,Bi3+ nanophosphors for thermometry applications†
Abstract
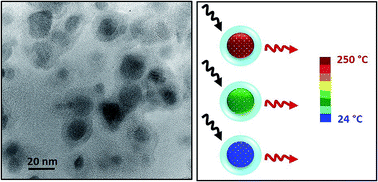
Chromium(III) and bismuth(III) co-doped ZnGa2O4 nanoparticles are synthesized by a hydrothermal method assisted by microwave heating. The obtained nanoparticles, with a diameter smaller than 10 nm, present good luminescence emission in the deep red range centered at 695 nm after coating with a silica layer and calcination at 1000 °C during 2 h. Persistent luminescence and photoluminescence properties are investigated at several temperatures. Bandwidth and luminescence intensity ratio of persistent emission do not present enough change with temperature to obtain a competitive nanothermometer with high sensitivity. Nevertheless, persistent luminescence decay curves present a significant shape change since the trap levels involved in the deexcitation mechanism are unfilled with increase of temperature. Even if the sensitivity reaches 1.7% °C−1 at 190 °C, the repeatability is not optimal. Furthermore, photoluminescent lifetime in the millisecond range extracted from the photoluminescence decay profiles drastically decreases with temperature increase. This variation is attributed to the thermal equilibrium between two thermally coupled chromium(III) levels (2E and 4T2) that have very different deexcitation lifetimes. For ZnGa2O4:Cr3+0.5%,Bi3+0.5%, the temperature sensitivity reaches 1.93% °C−1 at 200 °C. Therefore, this kind of nanoparticle is a very promising thermal sensor for temperature determination at the nanoscale.


 Please wait while we load your content...
Please wait while we load your content...