Substrate switchable Suzuki–Miyaura coupling for benzyl ester vs. benzyl halide†
Abstract
Two reaction conditions were developed to accomplish the substrate switchable Suzuki–Miyaura coupling of benzyl derivatives and arylboronic acid derivatives. Under conditions for esters, benzyl esters such as carbonates and acetates reacted with arylboronic acids to afford the corresponding diarylmethanes. However, the benzyl halides did not react under the same conditions. On the other hand, benzyl halides such as bromides and chlorides furnished diarylmethanes under conditions for halides, under which benzyl ester substrates did not react, in which water was found to play an important role. This switching system was tested using the intermolecular/intramolecular competitive reactions, during which the desired products could be synthesized by selecting the appropriate reaction conditions.
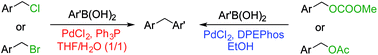


 Please wait while we load your content...
Please wait while we load your content...