Synthesis of α,β-unsaturated esters of perfluoropolyalkylethers (PFPAEs) based on hexafluoropropylene oxide units for photopolymerization†
Abstract
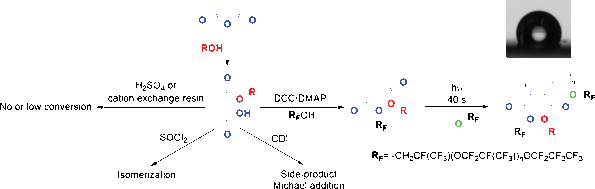
α,β-unsaturated esters are usually synthesized for polymer applications. However, the addition of maleate (cis-configuration) to a fluorinated moiety is challenging due to its potential isomerization during esterification. Various synthetic routes were attempted and led to very low conversion or side-products. The immiscibility of both reagents combined with an easy isomerization or attack on the double bond were potential explanations. In this paper, the synthesis of maleates oligo(hexafluoropropylene oxide) is reported by Steglich esterification and the reaction conditions are discussed depending on the molecular weight of the fluorinated moieties. After UV-curing, hydrophobic polymers were obtained by copolymerization with vinyl ethers by electron acceptor–donor systems.


 Please wait while we load your content...
Please wait while we load your content...