Pure phase synthesis of Cu3PS4 and Cu6PS5Cl for semiconductor applications†
Abstract
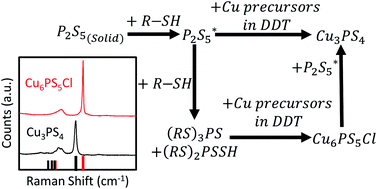
We have achieved the first reported pure phase synthesis of two new nanoparticle materials, Cu3PS4 and Cu6PS5Cl. We have achieved this through learning about the potential reaction pathways that CuCl2, P2S5, and 1-dodecanethiol can take. This study has shown that the key variable to control is the state of the phosphorus source when the CuCl2 is added. If P2S5 is added together with the CuCl2 to dodecanethiol then the reaction will follow a path to Cu3PS4, but if it is dissolved in dodecanethiol prior to the addition to CuCl2 then the reaction will produce Cu6PS5Cl. The formation of these two different phases can occur simultaneously, yet we have found sets of conditions that manipulate the reaction system to form each phase exclusively. These nanoparticles could have broad semiconductor or solid electrolyte applications.


 Please wait while we load your content...
Please wait while we load your content...