Alginic acid aquagel as a template and carbon source in the synthesis of Li4Ti5O12/C nanocomposites for application as anodes in Li-ion batteries†
Abstract
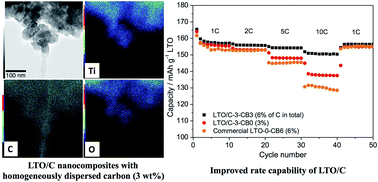
We report here a simple process for the synthesis of Li4Ti5O12(LTO)/carbon nanocomposites by a one-pot method using an alginic acid aquagel as a template and carbon source, and lithium acetate and TiO2 nanoparticles as precursors to the LTO phase. The carbon content can be tuned by adjusting the relative amount of alginic acid. The obtained materials consist of nanosized primary particles of LTO (30 nm) forming micron-sized aggregates covered by well-dispersed carbon (from 3 to 19 wt%). The homogeneous dispersion of carbon over the particles improves the electrochemical performance of LTO electrodes such as rate capability (>95 mA h g−1 at 40C) and cycling performance (>98% of retention after 500 cycles at 5C), even with only 3% of carbon black additive in the electrode formulation. With a simple and easily up-scalable synthesis, the LTO/carbon nanocomposites of this study are promising candidates as anode materials for practical application in lithium-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...