One-step preparation of novel 1-(N-indolyl)-1,3-butadienes by base-catalysed isomerization of alkynes as an access to 5-(N-indolyl)-naphthoquinones†
Abstract
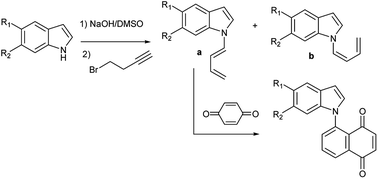
A series of novel 1-(N-indolyl)-1,3-butadienes, as (1 : 1) mixtures of the (E) and (Z) dienes, was prepared in one step by base-catalysed isomerization of N-alkylindoles with a terminal butyne chain. The reaction conditions are mild, and in all cases the yields were very high (>90%). The (E) and (Z) dienes were separable by preparative TLC and could be fully characterized. This isomerization proceeded readily in the case of a butynyl chain, but didn't take place with a pentynyl chain. A mechanism was proposed for this reaction, based on previous studies on the isomerization of alkynes in basic media, and a key intermediate that supports the proposed mechanism could be isolated and fully characterized. A theoretical study of the proposed mechanism was performed by computational methods and the results validated the proposal. The reactivity of the synthesized dienes was studied in Diels–Alder reactions with p-benzoquinone, to obtain a small library of new 5-(N-indolyl)-1,4-naphthoquinones.The lack of reactivity in the case of the (Z) isomers was explained by calculation of the rotational curves of the central bond of the (Z) and (E) dienes. Finally, the cytotoxicity of the new 5-(N-indolyl)-1,4-naphthoquinones was tested against a panel of three cell lines.


 Please wait while we load your content...
Please wait while we load your content...