Quinic acid and hypervalent chromium: a spectroscopic and kinetic study†
Abstract
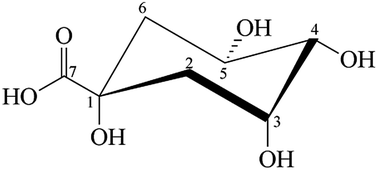
The redox reaction between an excess of quinic acid (QA) and CrVI involves the formation of intermediates, namely, CrIV and CrV species, which in turn react with the organic substrates. As observed with other substrates that have already been studied, CrIV does not accumulate during this reaction because of the rate of the reaction. Its rate of disappearance is several times higher than that of the reaction of CrVI or CrV with QA. Kinetic studies indicate that the redox reaction proceeds via a combined mechanism that involves the pathways CrVI → CrIV → CrII and CrVI → CrIV → CrIII, which is supported by the observation of superoxo-CrIII (CrO22+) ions, free radicals, and oxo-CrV species as intermediates and the detection of CrVI ester species. The present study reports the complete rate laws for the QA/chromium redox reaction.


 Please wait while we load your content...
Please wait while we load your content...