Combining transition metals and transient directing groups for C–H functionalizations
Abstract
In the domain of synthetic chemistry, C–H bond activation has always remained in the spotlight for researchers over the last few decades. Although different strategies have been employed to chemically trigger unactivated C–H bonds, transition metal catalyzed directing group (DG) aided C–H bond activation is the most explored pathway of all because of its ability to perform diverse site selective functional metamorphosis. Despite its popularity, tedious synthetic methodology requiring additional steps for the installation and removal of DGs from the target substrate diminishes its efficacy. However, replacement of directing groups by transient directing groups (tDGs) reduces the hurdle to a greater extent without compromising the product yield and selectivity. In this report we have depicted the intense journey of transient directing groups with three (Rh, Ru, and Pd) prevalent second row transition metals.
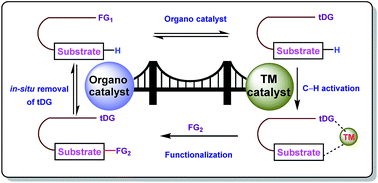
- This article is part of the themed collections: Organic chemist’s toolbox, Editors’ collection: Catalytic Organic Transformations and Chemical Frontiers Goa


 Please wait while we load your content...
Please wait while we load your content...