A new efficient domino approach for the synthesis of pyrazolyl-phthalazine-diones. Antiradical activity of novel phenolic products†
Abstract
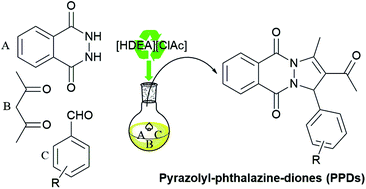
Pyrazolyl-phthalazine-dione derivatives (PPDs) were synthetized in the ionic liquid catalyzed one-pot multicomponent reaction of acetylacetone, 2,3-dihydrophthalazine-1,4-dione, and different aldehydes in moderate to good yields. Six new PPDs were obtained, and the crystal structure of 2-acetyl-1-(4-fluorophenyl)-3-methyl-1H-pyrazolo[1,2-b]phthalazine-5,10-dione (PPD-4) was determined. The most interesting structural features of the novel PPD-4 is the formation of a rather short intermolecular distance between the F atom of one molecule and the midpoint of the neighbouring six-membered heterocyclic ring. This interaction arranges all molecules into parallel supramolecular chains. UV-Vis spectra of all PPDs were acquired and compared to the simulated ones obtained with TD-DFT. All synthetized compounds were subjected to evaluation of their in vitro antioxidative activity using a stable DPPH radical. It was shown that PPD-7, with a catechol motive, is the most active antioxidant, while PPD-9, with two neighbouring methoxy groups to the phenolic OH, exerted a somewhat lower, but significant antioxidative potential. The results of DFT thermodynamical study are in agreement with experimental findings that PPD-7 and PPD-9 should be considered as powerful radical scavengers. In addition, the obtained theoretical results (bond dissociation and proton abstraction energies) specify SPLET as a prevailing radical scavenging mechanism in polar solvents, and HAT in solvents with lower polarity. On the other hand, the obtained reaction enthalpies for inactivation of free radicals suggest competition between HAT and SPLET mechanisms, except in the case of the ˙OH radical in polar solvents, where HAT is labeled as prefered.


 Please wait while we load your content...
Please wait while we load your content...