Adsorption of Cu(ii), Zn(ii), and Pb(ii) from aqueous single and binary metal solutions by regenerated cellulose and sodium alginate chemically modified with polyethyleneimine†
Abstract
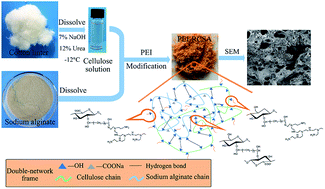
In this paper, crosslinked cellulose/sodium alginate (SA) was modified with polyethyleneimine (PEI) as an adsorbent (PEI-RCSA) for comparative and competitive adsorption of Cu(II), Zn(II), and Pb(II) in single and binary aqueous solutions. FTIR, SEM, TGA and specific surface area analysis were used to characterize the structural characteristics of PEI-RCSA. The effects of initial pH of solutions, contact time and initial concentration of heavy metal ions on the adsorption capacity of PEI-RCSA were investigated. The experimental results revealed that the removal of metal ions on the PEI-RCSA was a pH-dependent process with the maximum adsorption capacity at the initial solution pH of 5–6. The adsorption kinetics were followed by a pseudo-second-order kinetics model, and the diffusion properties played a significant role in the control of the adsorption kinetics. Meanwhile, adsorption isotherms were successfully described by the Langmuir model in a single aqueous solution system. The maximum adsorption capacities of PEI-RCSA for Cu(II), Zn(II), and Pb(II) in a single system were 177.1, 110.2 and 234.2 mg g−1, respectively. The binary-component system was better described with the Langmuir competitive isotherm model. The removal efficiencies didn't change significantly when three adsorption–desorption experimental cycles were conducted. All the above results indicated that PEI-RCSA has promising applications in the treatment of toxic metal pollution.


 Please wait while we load your content...
Please wait while we load your content...