Metal-free C–H arylation of imidazoheterocycles with aryl hydrazines†
Abstract
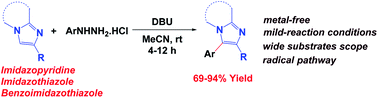
A simple and efficient metal-free arylation of imidazo[1,2-a]pyridines at the C-3 position with arylhydrazine has been achieved at room temperature under ambient air conditions. Various 2,3-disubstituted imidazopyridines and imidazothiazoles were synthesized with high yields. The present methodology demonstrates the usefulness of commercially available aryl hydrazine as an arylating agent.


 Please wait while we load your content...
Please wait while we load your content...