Radical-initiated alkene hydroauration as a route to gold(iii) alkyls: an experimental and computational study†
Abstract
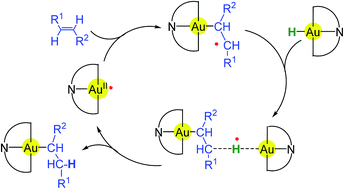
The hydroauration of functionalised 1-alkenes by the gold(III) hydride (C^NOMe^C)AuH is initiated by organic radicals and proceeds via (C^N^C)Au(II) radical intermediates following a bimolecular outer-sphere mechanism. The outcome of these reactions is determined by the stability of the gold-substituted radicals, and chemoselectivity correlates with the degree of spin delocalisation in the alkylgold radical intermediates. The reaction is sensitive to steric as well as electronic factors; disubstituted alkenes and alkenes that form unstable radicals give product mixtures or are unreactive. As DFT calculations show, the reactions agree well with the calculated reaction enthalpies and the standard free energy change for the reaction of the gold(II) radical with the respective alkene.


 Please wait while we load your content...
Please wait while we load your content...