Electronic structure, polaron formation, and functional properties in transition-metal tungstates
Abstract
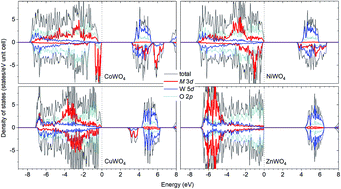
Transition-metal tungstates MWO4 (M = Co, Ni, Cu, Zn) have applications in many areas, including supercapacitors. A good understanding of the electronic structure is essential to understanding their functional properties. Here, we report a first-principles study of the materials using hybrid density-functional calculations. The electronic structure is analyzed with a focus on the nature of the electronic states near the band edges. We find that hole polarons can form at the Co lattice site in CoWO4 and the O site in NiWO4, CuWO4, and ZnWO4, resulting in the formation of Co3+ in the former and O− in the latter. The electrochemical activity observed in certain tungstate compounds, but not in others, appears to be related to the ability to form hole polarons on the transition-metal ions. The formation energy and migration barrier of the hole polaron in CoWO4 are also calculated and the results are employed to understand the reported p-type conductivity.


 Please wait while we load your content...
Please wait while we load your content...