Effect of relative humidity on the gas transport properties of zeolite A/PTMSP mixed matrix membranes†
Abstract
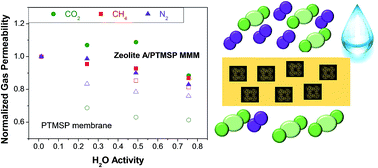
Increasing the knowledge of the influence of water vapor in new mixed matrix membranes (MMMs) could favor the integration of novel membrane materials in the recovery of CO2 from wet industrial streams. In this work, the water vapor effect on the N2, CH4 and CO2 permeability through MMMs comprised of 20 wt% hydrophilic zeolite 4A in hydrophobic PTMSP polymer were investigated in the relative humidity range 0–75%. While in the pure PTMSP membranes, the permeability of all gases decreases with water vapor activity, with almost unchanged CO2/N2 and CO2/CH4 selectivities, in zeolite A/PTMSP MMMs, the CO2 permeability increases with increasing water content in the system up to 50% R.H., resulting in an increase in CO2/N2 and CO2/CH4 selectivities with respect to pure PTMSP. Gas sorption was studied so that the effect the residual humidity in the zeolite 4A has on the sorption of the different gases helped explaining the permeability observations. The sorption and humid permeation behavior were evaluated by a simple model equation based on the NELF theory, taking into account the multicomponent gas sorption and diffusion in the presence of humidity, as well as the counteracting effects of the hydrophobic PTMSP and hydrophilic zeolite A in a very accurate way.
- This article is part of the themed collection: Zeolites and 3D Porous Solids


 Please wait while we load your content...
Please wait while we load your content...