Self-exfoliation of 2D covalent organic frameworks: morphology transformation induced by solvent polarity†
Abstract
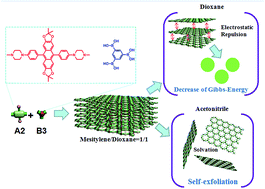
Recently, covalent organic nanosheets (CONs) have emerged as functional two-dimensional (2D) materials for versatile applications. Strong interaction among layers and the instability of borate ester in moisture are the major hurdles to obtain few layered boron-containing CONs by exfoliation of their bulk counterparts. In this paper, we report a facile approach for preparation of few layered borate ester-containing CONs based on electrostatic repulsion of ions. We incorporated organic ionic groups into porous covalent organic frameworks (COFs) and it has been proved that the COFs with quaternary ammonium group could self-exfoliate into few layered ionic covalent organic nanosheets (iCONs) in polar organic solvents. Interestingly, the morphology of the iCOFs-A could be changed from a multilayered aggregation to nanocapsules, or 2D sheets when solvents with different polarity were used. In contrast, non-ionic covalent organic frameworks COFs-B could not self-exfoliate in various solvents. In addition, the self-exfoliated nanosheets could be used to fabricate uniform thin films on SiO2 wafer and the film exhibited explicit optical and electrical properties.


 Please wait while we load your content...
Please wait while we load your content...