Approach to pactamycin analogues using rhodium(ii)-catalyzed alkene aziridination and C(sp3)–H amination reactions†
Abstract
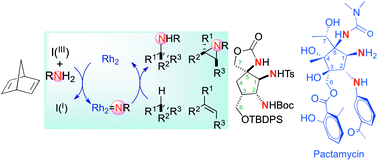
Dirhodium(II)-catalyzed nitrene transfers have been used to prepare a novel synthetic platform bearing the triamino moiety present in pactamycin, jogyamycin and cranomycin. Catalytic intramolecular C(sp3)–H amination and alkene aziridination reactions, the latter followed by a nucleophilic aziridine ring opening, are the key steps of this strategy.
- This article is part of the themed collection: Synthetic Approaches to Natural Products via Catalytic Processes


 Please wait while we load your content...
Please wait while we load your content...