Identification of the key factor promoting the enrichment of chiral polymorph A in zeolite beta and the synthesis of chiral polymorph A highly enriched zeolite beta†
Abstract
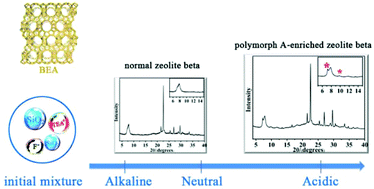
Chiral zeolites with intrinsically chiral frameworks are highly desired due to their potential applications in asymmetric catalysis and enantioseparation. Zeolite beta is an intergrowth of chiral polymorph A and achiral polymorph B in a ratio of ca. 44 : 56. The synthesis of chiral polymorph A of zeolite beta or chiral polymorph A-enriched zeolite beta is one of the biggest challenges. By performing a series of control experiments, we identified the key factor promoting the enrichment of chiral polymorph A in pure-silica zeolite beta, i.e. the acidity of the initial mixture. With this discovery, we developed a generalized non-hydrofluoric acid-assisted synthesis route to synthesize polymorph A-enriched pure-silica zeolite beta. Zeolite beta crystals containing more than 70% chiral polymorph A were obtained. This acid assisted method could not only significantly enrich polymorph A in the crystals of pure-silica zeolite beta but also provide a way for avoiding the utilization of highly toxic concentrated hydrofluoric acid.
- This article is part of the themed collections: Inorganic Chemistry Frontiers HOT articles for 2018 and In honour of Professor Xu Ruren for his forty-year contribution in zeolitic materials research


 Please wait while we load your content...
Please wait while we load your content...