Converting natural rubber waste into ring-opening metathesis polymers with oligo-1,4-cis-isoprene sidechains†
Abstract
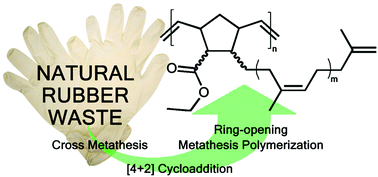
A chemical recycling of natural rubber waste via a degradation/polymerisation approach is described. The vulcanized rubber waste was degraded by cross metathesis with ethyl acrylate as the key-step yielding enoate end-capped oligo-cis-isoprenes, which were subsequently converted into norbornenes via a cycloaddition reaction with cyclopentadiene. Ring-opening Metathesis Polymerisation (ROMP) then yielded main-chain unsaturated polymers bearing oligo-1,4-cis-isoprene side chains with appealing thermal stability and a glass transition temperature of −60 °C.


 Please wait while we load your content...
Please wait while we load your content...