Aza-tricycles containing a perfluoroalkyl group: synthesis, structure and fluorescence†
Abstract
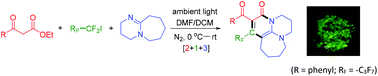
Perfluoroalkyl-containing aza-tricycles have been prepared in one synthetic operation via an ambient light-promoted three-component reaction of β-oxo esters, perfluoroalkyl iodide and DBU. Intramolecular C–F⋯O and double C–H⋯F weak interactions and intermolecular C–H⋯O and C–H⋯π hydrogen bondings were observed partly due to the incorporation of the perfluoroalkyl group. The perfluoroalkylated non-planar aza-tricycles exhibit interesting room-temperature AIE fluorescence and acid-induced fluorescence enhancement characters.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...