Synthesis and application of a highly branched, mechanism-based 2-deoxy-2-fluoro-oligosaccharide inhibitor of endo-xyloglucanases†
Abstract
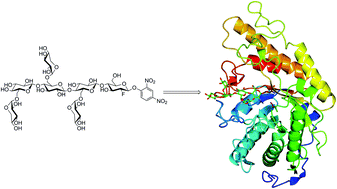
Xyloglucan (XyG) is a complex polysaccharide that is ubiquitous and often abundant in the cell walls of terrestrial plants. XyG metabolism is therefore a key component of the global carbon cycle, and hence XyG enzymology is of significant fundamental and applied importance in biomass conversion. To facilitate structure–function analyses of XyG-specific endo-glucanases, we have synthesized a 2′,4′-dinitrophenyl 2-deoxy-2-fluoro-β-glycoside mechanism-based inhibitor based on the highly branched XyG repeating motif XXXG (Xyl3Glc4: ([α-D-Xylp-(1→6)]-β-D-Glcp-(1→4)-[α-D-Xylp-(1→6)]-β-D-Glcp-(1→4)-[α-D-Xylp-(1→6)]-β-D-Glcp-(1→4)-D-Glcp. Key steps in the chemo-enzymatic synthesis included selective enzyme hydrolysis of XyG polysaccharide to produce the core heptasaccharide, per-O-acetylation, α-bromination, reductive glycal formation, electrophilic fluorination, SNAr glycosylation, and Zemplen deprotection. The resulting compound, XXXG(2F)-β-DNP, specifically labelled the active sites of several endo-(xylo)glucanases by accumulation of a covalent glycosyl-enzyme intermediate, as revealed by intact protein mass spectrometry. Crystallography of a complex with a Cellvibrio japonicus Glycoside Hydrolase Family 5 (GH5) endo-xyloglucanase corroborated the covalent nature of the intermediate, and further revealed the anticipated specificity for the catalytic nucleophile of this anomeric-configuration-retaining glycosidase. This specificity complements that of an analogous XXXG N-bromoacetylglycosylamine inhibitor, which labelled the catalytic acid–base sidechain in the same enzyme [Attia, et al., Biotechnol. Biofuels, 2018, 11, 45]. We anticipate that these inhibitors may find continued use in mechanistic analyses of endo-(xylo)glucanases from diverse GH families.
- This article is part of the themed collections: Chemical Biology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...