Expedient synthesis of trifunctional oligoethyleneglycol-amine linkers and their use in the preparation of PEG-based branched platforms†
Abstract
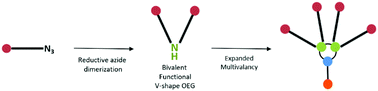
We designed a convergent synthesis pathway that provides access to trifunctional oligoethyleneglycol-amine (OEG-amine) linkers. By applying the reductive coupling of a primary azide to bifunctional OEG-azide precursors, the corresponding symmetrical dialkylamine bearing two homo-functional end chain groups and a central nitrogen was obtained. These building blocks bear minimal structural perturbation compared to the native OEG backbone which makes them attractive for biomedical applications. The NMR investigations of the mechanism process reveal the formation of nitrile and imine intermediates which can react with the reduced free amine form. Additionally, these trifunctional OEG-amine linkers were employed in a coupling reaction to afford branched multifunctional PEG dendrons which are molecularly defined. These discrete PEG-based dendrons (n = 16, 18 and 36) could be useful for numerous applications where multivalency is required.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...