An experimental and computational study on isomerically pure, soluble azaphthalocyanines and their complexes and boron azasubphthalocyanines of a varying number of aza units†
Abstract
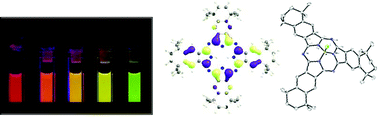
Herein, we present a series of isomerically pure, peripherally alkyl substituted, soluble and low aggregating azaphthalocyanines as well as their new, smaller hybrid homologues, azasubphthalocyanines. The focus lies on the effect of the systematically increasing number of aza building blocks [–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ] replacing the non-peripheral [–CH
] replacing the non-peripheral [–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ] units and their influence on the physical and photophysical properties of these chromophores. The absolute and relative HOMO–LUMO energies of azaphthalocyanines were analyzed using UV-Vis and CV and compared to the density functional theory calculations (B3LYP, TD-DFT). The lowering of the HOMO level is revealed as the determining factor for the trend in the adsorption energies by electronic structure analysis. Crystals of substituted subphthalocyanines, N2-Pc*H2 and N4-[Pc*Zn·H2O], were obtained out of DCM. For the synthesis of the valuable tetramethyltetralin phthalocyanine building block a new highly efficient synthesis involving a nearly quantitative CoII catalyzed aerobic autoxidation step is introduced replacing inefficient KMnO4/pyridine as the oxidant.
] units and their influence on the physical and photophysical properties of these chromophores. The absolute and relative HOMO–LUMO energies of azaphthalocyanines were analyzed using UV-Vis and CV and compared to the density functional theory calculations (B3LYP, TD-DFT). The lowering of the HOMO level is revealed as the determining factor for the trend in the adsorption energies by electronic structure analysis. Crystals of substituted subphthalocyanines, N2-Pc*H2 and N4-[Pc*Zn·H2O], were obtained out of DCM. For the synthesis of the valuable tetramethyltetralin phthalocyanine building block a new highly efficient synthesis involving a nearly quantitative CoII catalyzed aerobic autoxidation step is introduced replacing inefficient KMnO4/pyridine as the oxidant.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...