Stepwise triple-click functionalization of synthetic peptides†
Abstract
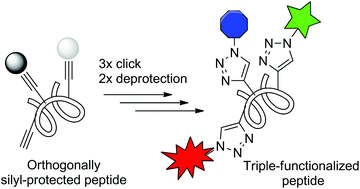
The increasing popularity of peptides as promising molecular scaffolds for biomedical applications and as valuable biochemical probes makes new methods allowing for their modification highly desirable. We describe herein an optimized protocol based on a sequence of CuAAC click reactions and selective deprotection steps, which leads to an efficient multi-functionalization of synthetic peptides. The methodology has been successfully applied to the construction of defined heteroglycopeptides and fluorophore–quencher-containing probes for proteases. The developed chemistry thus represents an important addition to the available toolbox of methods enabling efficient postsynthetic modification of peptides. The commercial availability of numerous azide probes further greatly extends the application potential of the described methodology.


 Please wait while we load your content...
Please wait while we load your content...