trans-Hydroboration vs. 1,2-reduction: divergent reactivity of ynones and ynoates in Lewis-base-catalyzed reactions with pinacolborane†
Abstract
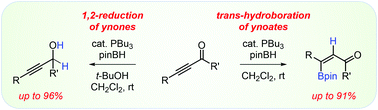
Ynones and ynoates react with pinacolborane in a divergent manner in the presence of nucleophilic phosphine catalysts. Ynones are transformed to the corresponding propargyl alcohols in good yields with high regio- and chemoselectivity. Ynoates undergo highly regio- and stereoselective trans-hydroboration to produce E-vinylboronates. Impressive divergence in reactivity of ynones and ynoates can be traced back to the mechanistic aspects of 1,2-reduction and trans-hydroboration. A comparative analysis of the two pathways paints a complex picture in which different reaction rates control selectivity in these seemingly unrelated processes and explains how sufficiently acidic protons in the reaction mixtures can be used to steer the selectivity in different directions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...