Modular synthesis of heptaarylindole†
Abstract
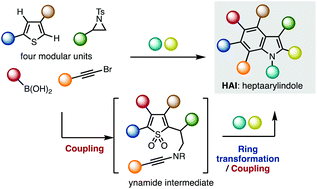
The first synthesis of heptaarylindole (HAI) has been accomplished using a coupling/ring transformation strategy. Four readily prepared modular units (diarylthiophenes, 2-arylaziridines, arylboronic acids, and arylalkynes) were joined together to provide key ynamide intermediates. Subsequent inverse electron-demand intramolecular [4 + 2] cycloaddition furnished pentaarylindoles (PAIs) regioselectively. This strategy was also applied to the synthesis of tetraarylazaindole with four different aryl substituents. PAIs underwent further arylations at the C2- and N1-positions, providing HAI with seven different aryl substituents with virtually complete regioselectivity.


 Please wait while we load your content...
Please wait while we load your content...