Synthesis and structural elucidation of a dehydrochloromethyltestosterone metabolite†
Abstract
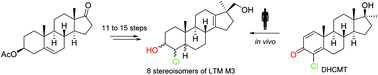
The human urinary long-term metabolite “M3” (4-chloro-17β-hydroxymethyl-17α-methyl-18-norandrost-13-en-3-ol) of the common doping agent DHCMT has thus far been detected via GC/MS-MS, creating ambiguities concerning its absolute configuration. Its structure was elucidated via the synthesis of all eight possible stereoisomers with 17β-hydroxymethyl configuration. The highlights of the synthesis consist of a novel first generation approach to 4β-chloro-5β compounds as well as a divergent route which allows easy access to the remaining A-ring chlorohydrins.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...