gem-Diborylalkanes: recent advances in their preparation, transformation and application
Abstract
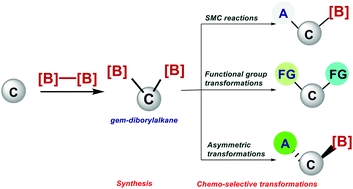
Recently, gem-diborylalkanes have attracted much attention as versatile building blocks and fundamental intermediates in organic synthesis, because they enable multiple C–C bond construction and further transformation at C–B bonds. Importantly, gem-diborylalkanes can be utilised as bisnucleophilic partners in a variety of chemo-selective C–C bond-forming reactions. This review describes recent developments in synthesising gem-diborylalkanes in complex molecules along with their chemical transformation. In the first part of the review the different synthetic approaches used to synthesise gem-diborylalkanes are described. In the second part, an overview of the chemoselective transformation of gem-diborylalkanes into various functionalized materials is discussed along with one-carbon homologation of diborylmethane via a selective uni- and bidirectional method.


 Please wait while we load your content...
Please wait while we load your content...