Solid-state nanopore analysis on conformation change of p53TAD–MDM2 fusion protein induced by protein–protein interaction†
Abstract
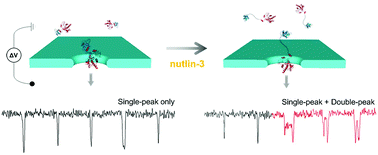
Although protein–protein interactions (PPIs) are emerging therapeutic targets for human diseases, development of high-throughput screening (HTS) technologies against PPI targets remains challenging. In this study, we propose a protein complex structure to effectively detect conformational changes of protein resulting from PPI using solid-state nanopore for a novel, widely-applicable drug screening method against various PPI targets. To effectively detect conformational changes resulting from PPI, we designed a fusion protein MLP (MDM2-linker-p53TAD), where p53TAD and MDM2 are connected by a 16 amino acid linker. The globular conformation of MLP exhibited a single-peak translocation event, whereas the dumbbell-like conformation of nutlin-3-bound MLP revealed as a double-peak signal. The proportion of double-peak to single-peak signals increased from 9.3% to 23.0% as nutlin-3 concentration increased. The translocation kinetics of the two different MLP conformations with varied applied voltage were analyzed. Further, the fractional current of the intra-peak of the double-peak signal was analyzed, probing the structure of our designed protein complex. This approach of nanopore sensing may be extendedly employed in screening of PPI inhibitors and protein conformation studies.


 Please wait while we load your content...
Please wait while we load your content...