On the nature of the boron–copper interaction. Topological study of the electron localisation function (ELF)†
Abstract
Topological analysis of the Electron Localization Function (ELF) has been applied to study the nature of the boron–copper bonds in the BCu molecule and a series of 18 organoboron compounds with bond lengths between 1.994 and 2.671 Å. The three-center and two-center covalent bonds have been found through localisation of the V(B,Cu,A), A = Mn, Cu, B, H and C, and V(B,Cu) attractors with the basin populations from 1.49 to 3.13e and from 2.49 to 2.99e, respectively. For ropt(B,Cu) > 2.5 Å, B⋯Cu interactions, characterised by very small basin populations, between 0.12 and 0.49e have been observed. Topological analysis of ELF has not proven the existence of a triple B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Cu bond.
Cu bond.
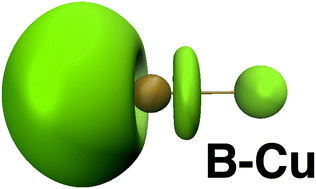


 Please wait while we load your content...
Please wait while we load your content...