Lemon juice mediated multicomponent reactions for the synthesis of fused imidazoles†
Abstract
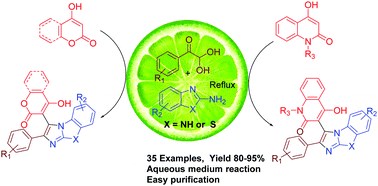
A one-pot three component reaction mediated by lemon juice in an aqueous medium for the synthesis of diverse pharmaceutically important tricyclic fused imidazoles tethered with aryl and various cyclic 1,3-dicarbonyls has been reported. The reaction of arylglyoxals with cyclic 1,3-diacarbonyls such as N-methyl-4-hydroxyquinolone, 2,4-dihydroxyquinoline, 4-hydroxycoumarin or 4-hydroxy-6-methyl-2-pyrone with various 1,3-N,N-binucleophiles such as 2-aminobenzothiazoles or 2-aminobenzimidazoles provided medicinally important diverse tricyclic fused imidazoles having aryl and cyclic 1,3-dicarbonyl substituents under metal-free conditions. This method is a natural acid catalyzed multicomponent reaction in an aqueous medium for the synthesis of diverse tricyclic fused imidazoles in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...