Pyrenyl substituted 1,8-naphthalimide as a new material for weak efficiency-roll-off red OLEDs: a theoretical and experimental study†
Abstract
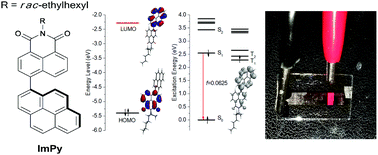
Based on the theoretical calculations of excited states and semiconducting properties, a new 1,8-naphthalimide derivative having an electron-donating 1-pyrenyl group at the C-4 position was designed and synthesized. This derivative exhibited an excellent thermal stability and bipolar charge carrier transport ability. It was successfully utilized as a host in red phosphorescent organic light-emitting diodes showing an efficient energy transfer from the host to the phosphorescent emitter. The derivative may be a single material electroplex-forming host for PhOLEDs. The best fabricated red emitting device demonstrated maximum current, power, and external quantum efficiencies of 10.8 cd A−1, 7 lm W−1, and 13.6%, respectively. The best device exhibited a high brightness of 15 300 cd m−2 and weak efficiency-roll-off.


 Please wait while we load your content...
Please wait while we load your content...