A long-range tautomeric effect on a new Schiff isoniazid analogue, evidenced by NMR study and X-ray crystallography†
Abstract
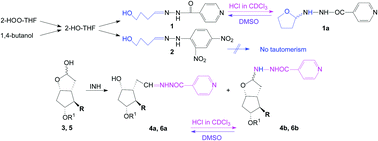
Long-range tautomerism to a N,O-aminal thereby closing a tetrahydrofuran ring was evidenced for an isoniazid analogue, whose accidental synthesis is presented in the paper. The isoniazid analogue was synthesized by the reaction of isoniazid with 2-hydroxy-tetrahydrofuran which was demonstrated to exist in old THF together with other peroxides, especially 2-HOO-THF. The same compound was efficiently obtained from a THF containing 2-HOO-THF, by reducing this peroxide in the presence of isoniazid. The 2,4-dinitrophenylhydrazone was also synthesized. The oxidation of 1,4-butanediol and the reaction of the resulting mono-aldehyde with isoniazid gave the same compound. The existence of the linear tautomer was evidenced in the NMR spectra in DMSO-d6 and was confirmed by X-ray analysis to be the single tautomer in the crystal. The cyclic N,O-aminal tautomer was found in the NMR spectra in CDCl3, resulting from an intramolecular HCl-catalyzed addition of the hydroxyl group to the double bond CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N of the linear tautomer, thereby closing a tetrahydrofuran ring. This is a favoured cyclization according to Baldwin's rules (5-exo-trig). The same tautomerism was also present for two isoniazid analogues obtained from two lactols, used in prostaglandin synthesis. The compounds 1, 4, 6 and INH had no antibacterial or antifungal activity.
N of the linear tautomer, thereby closing a tetrahydrofuran ring. This is a favoured cyclization according to Baldwin's rules (5-exo-trig). The same tautomerism was also present for two isoniazid analogues obtained from two lactols, used in prostaglandin synthesis. The compounds 1, 4, 6 and INH had no antibacterial or antifungal activity.


 Please wait while we load your content...
Please wait while we load your content...